
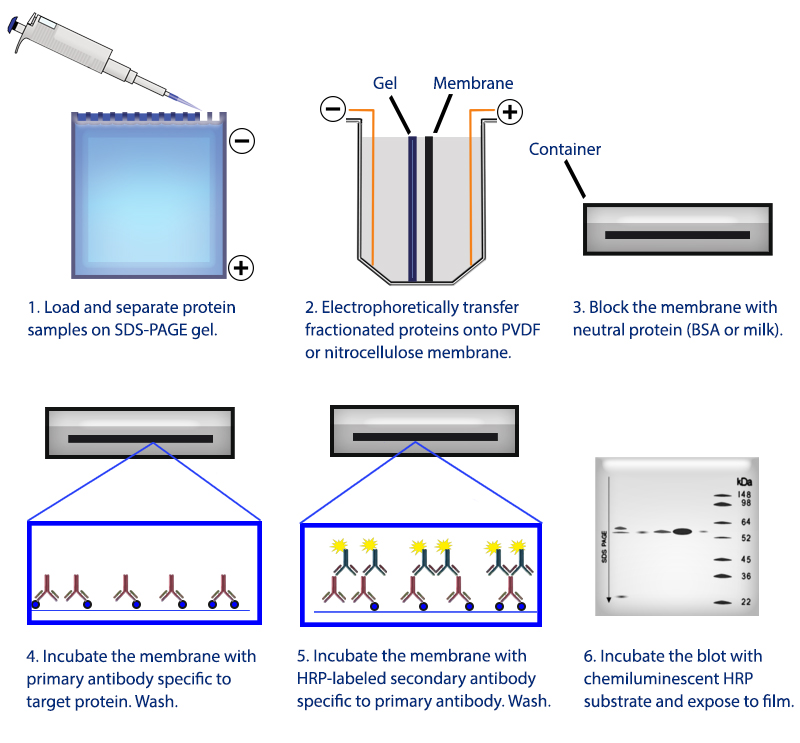
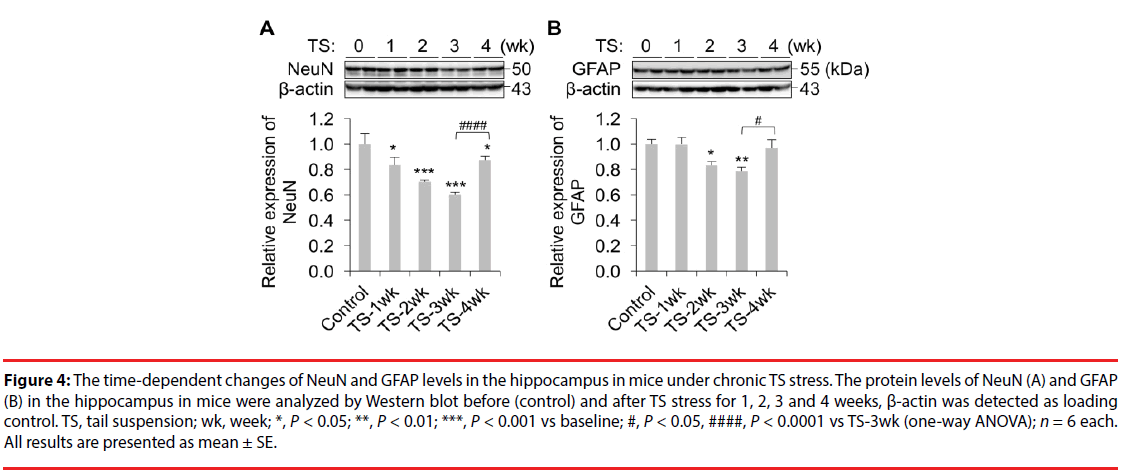
Moving the resolved proteins sample to nitrocellulose provides a durable substrate which may then be probed. Once the proteins in the sample have been sufficiently separated by their molecular weight, they are then ready for transfer. Larger proteins are retarded and remain closer to the loading wells at the top of the gel while smaller proteins travel further as the migration front, potentially off the gel if proteins are small and run long enough. Higher polyacrylamide percentage yields decreased pore sizes while increasing cross-linker percentage yields larger pore sizes. As they travel, proteins are restricted by pores whose size is determined by the polyacrylamide and cross-linker percentage of the gel. These negatively charged, linearized proteins then migrate away from the cathode, the negatively charged area near the loading region of the gel, and towards the anode. SDS both denatures folded proteins, allowing them to travel more easily through the gel, as well as impart a negative charge to them. For proteins with molecular weights of 25 kDa, Mouse Anti-Rabbit IgG (Conformation Specific) (L27A9) mAb #3678 is recommended.Specifically in a Western Blot, samples of interest are loaded into a well of polyacrylamide gel (PAG) containing sodium dodecyl sulfate (SDS), to which an electric field (E) is applied, hence "SDS-PAGE". NOTE: For proteins with molecular weights of 50 kDa, we recommend using Mouse Anti-Rabbit IgG (Light-Chain Specific) (L57A3) mAb #3677 or Mouse Anti-Rabbit IgG (Conformation Specific) (L27A9) mAb #3678 as a secondary antibody to minimize masking produced by denatured heavy chains. Proceed to step 1 of Immunoprecipitation.Transfer the supernatant to a fresh tube. Take 200 μl cell lysate and add to either Protein A or G agarose beads (20 μl of 50% bead slurry).Analyze sample by Western blotting (see Western Immunoblotting Protocol: Western BSA or Western Milk).Heat the sample to 95–100☌ for 2–5 minutes and microcentrifuge for 1 minute at 14,000 X g.Vortex, then microcentrifuge for 30 seconds. Resuspend the pellet with 20 μl 3X SDS sample buffer.Wash pellet five times with 500 µl of 1X cell lysis buffer. Incubate with gentle rocking for 1–3 hours at 4☌. Add either protein A or G agarose beads (20 μl of 50% bead slurry).Incubate with gentle rocking overnight at 4☌. Take 200 μl cell lysate and add primary antibody.Optional: It may be necessary to perform a lysate pre-clearing step to reduce non-specific binding to the Protein A/G agarose beads (See section below). If necessary, lysate can be stored at –80☌.

Microcentrifuge for 10 minutes at 14,000 X g, 4☌, and transfer the supernatant to a new tube.Sonicate samples on ice three times for 5 seconds each.Scrape cells off the plates and transfer to microcentrifuge tubes.
Remove PBS and add 0.5 ml ice-cold 1X cell lysis buffer to each plate (10 cm) and incubate the plates on ice for 5 minutes.To harvest cells under nondenaturing conditions, remove media and rinse cells once with ice-cold PBS.Treat cells by adding fresh media containing regulator for desired time. Use Protein A for rabbit IgG pull down and Protein G for mouse IgG pull down. Protein A or G Agarose Beads: (Protein A #9863) Please prepare according to manufacturer’s instructions.NOTE: Add 1 mM PMSF immediately prior to use. 1X Cell Lysis Buffer: ( #9803) 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM Sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na 3VO 4, 1 μg/ml Leupeptin.
NOTE: Prepare solutions with Milli-Q or equivalently purified water. For shorter assay times please try our Immunoprecipitation Protocol Utilizing Magnetic Separation / (For Analysis By Western Immunoblotting).


 0 kommentar(er)
0 kommentar(er)
